Developing a fruit-growing project in southern Chile is completely different from what happens in other production areas. Here, a warm humid temperate climate and volcanic soils dominate, which result in late production and risks of climatic events during critical phenological periods such as flowering, fruit set, and fruit ripening.
The soils of southern Chile are mainly Andisols, derived from pyroclastic volcanic materials, whose alteration has given rise to structurally disordered clays, mainly Allophane and Imogolite.
The surface horizons are characterized by porosity (60%-70% of bulk volume), permeability, friability, low bulk density (< 0.9 g cm-³), moderately acidic to acidic pH (5.2-6.0), high organic matter content (8-20%), and high phosphate retention capacity. These are variable charge soils with high cation and anion exchange capacity and a high percentage of alumina (aluminum oxide -Al2O³-). The physical properties of these soils are exceptional, although their fertility is limited, mainly due to high phosphorus retention and a tendency toward acidification.
Soil acidity and effects on fruit trees
Soil acidification naturally occurs when precipitation exceeds evapotranspiration, producing continuous leaching of bases (Ca2+, Mg2+, K+, Na2+). In Chile, acidic soils appear in the Maule region and increase in frequency and extent toward the south of the country as precipitation increases, reaching their maximum expression in the regions of Los Ríos and Los Lagos.
However, the soil acidification process can be accelerated by additional sources of acidity: mineralization of organic matter, root respiration and exudation, sulfur (S) oxidation, and ammonium (NH4+) oxidation, among others.
In this context, the continuous use of ammoniacal fertilizers and urea, common practices in fruit production systems in southern Chile, can accelerate soil acidification in the absence of an adequate management strategy.
Under acidic pH conditions, aluminum (Al) present in the soil as silicates and oxides is solubilized to form Al3+ ions, which are toxic to plants, depending on the species and even the variety. This is one of the main obstacles to the growth and productivity of crops in southern Chilean soils.
In these soils, there is a relationship between pH-H2O and Al+3 availability, in which Al+3 increases rapidly at pH-H2O values below 5.5, far exceeding the critical level of 0.1 cmol+ kg-1 defined for sensitive species such as cherry or European hazelnut.
 Image 1: Assay methodology. A. Surface application of one of the liming amendments. B. Soil sampling in discrete depth increments.
Image 1: Assay methodology. A. Surface application of one of the liming amendments. B. Soil sampling in discrete depth increments.
In fruit crops, the toxic effects of Al - such as inhibition of root and aerial growth, impairment of nutrient and water uptake, reduction in the number of flowers, yield, and fruit quality, and alterations in basic physiological and biochemical processes - have been reported in many species worldwide. These include cherry, European hazelnut, citrus, apple, blueberry, raspberry, grapevine, peach.
Specifically, in acidic volcanic soils of southern Chile, Bonomelli and Artacho (2021) demonstrated the negative effect of Al on macronutrient uptake (particularly Ca) and the growth of cherry trees. The negative effects on fine root growth were evident as early as the first season following planting and at relatively low levels of Al in the soil (0.12 cmol kg-1).
Similarly, a prospective study in a European hazelnut orchard on a young Andisol in the Araucanía region established a relationship between high acidity (high Al availability in the soil) and a significant reduction in yield.
Additionally, it was determined that Al reaches fruit tissues, reducing their commercial quality (Bonomelli et al., 2021). Therefore, soil acidity must be corrected to ensure successful establishment and sustained high productivity of cherry trees, European hazelnuts, and other orchards on acidic volcanic soils in southern Chile.
Diagnosis and management of soil acidity
Soil liming is the most effective practice to reduce soil acidity, increase the availability of basic cations (Ca2+, Mg2+), reduce toxic Al availability, and consequently increase crop yields. Lime materials mainly include hydroxides, oxides, carbonates, and silicates of Ca and Mg.
Their dissolution produces OH- and Ca2+ that react with H+ ions present in the soil solution and adsorbed onto soil colloids, raising soil pH and reducing Al toxicity through the precipitation of Al3+ into inert forms.
Available options include traditional agricultural lime (mainly composed of calcium carbonate), dolomitic lime (also containing magnesium carbonate), and dolomitic lime (also containing magnesium carbonate). Additionally, calcium hydroxides (hydrated lime) and calcium oxides (quicklime) have faster reaction times compared to carbonates but are more expensive and difficult to handle and apply due to their caustic nature.
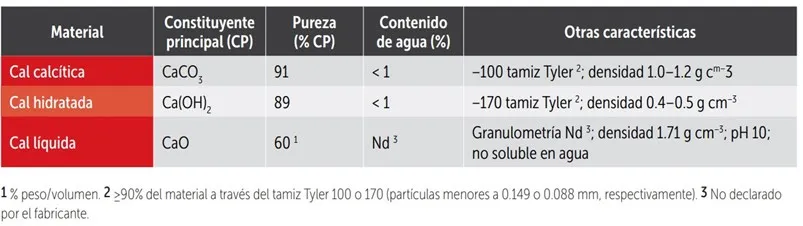 Table 1: Chemical and physical characteristics of the materials used in the experiment.
Table 1: Chemical and physical characteristics of the materials used in the experiment.
The effectiveness of a liming material in neutralizing soil acidity depends on its chemical composition, particularly purity, and its physical properties, especially fineness of grinding. The finer the particle size, the faster the product will react and neutralize soil acidity.
Both agricultural lime and dolomite have very low water solubility, so they move slowly through the soil profile, affecting only the immediate vicinity of the application. Therefore, lime applied on the surface without being incorporated into the soil is ineffective in correcting subsoil acidity.
Calcium oxides and hydroxides are more soluble in water, so they should react more quickly and move faster through the soil profile, although variable results have been reported in the literature.
Regardless of the material used, the liming rate must be calculated carefully, considering the crop requirements based on its tolerance to Al toxicity, the current state of soil acidity, the pH buffering capacity depending on the soil type, and the depth of tillage.
The application of excessive lime doses (over-liming) can significantly reduce micronutrient bioavailability and cause deficiencies in plants, as well as lead to nutritional imbalances and antagonism among cations during plant uptake.
Therefore, a decisive aspect is the diagnosis of soil acidity, which is carried out through soil analysis performed in a specialized and accredited laboratory, using acidity indicators such as soil pH, exchangeable Al, base saturation, and Al saturation. Exchangeable Al is a measure of Al+3 availability and varies with soil pH according to a known relationship. Therefore, pH reflects the content of exchangeable Al as well as measures the active acidity of the soil.
As a reference, 0.1 cmol Al kg-1 in soil has been defined as a critical level for sensitive plant species, so the pH of volcanic soils in southern Chile should be maintained near 5.8. Al saturation represents the proportion of cation exchange sites in the soil occupied by Al+3 that replace Ca+2 and Mg+2 cations. In this context, Miagri has set a limit of 5% Al saturation for regions with predominantly volcanic soils, above which there is a high probability of Al toxicity for crops.
Cherry orchard on acidic volcanic soil
The orchards, once established, function as no-tillage cultivation systems, sharing the challenge of managing subsoil acidity once the trees have been planted.
In the South of Chile, it is common to apply liming substances in an incorporated form before planting, with the effects of liming limited to the depth of soil cultivation. Subsequently, surface or blanket lime application becomes the only option to correct or maintain soil pH within optimal limits.
Therefore, it was hypothesized that continuous surface applications of lime, as a common management practice in orchards on acidic soils in southern Chile, could lead to excessive liming and base imbalances in the upper centimeters of the soil.
To test this hypothesis, a field trial was designed in a southern Chilean cherry orchard, evaluating the effectiveness of different liming materials in neutralizing soil acidity in terms of reaction rate and mobility within the soil profile [agricultural lime, calcium hydroxide (hydrated lime), and a commercial lime suspension (liquid lime)] with the aim of identifying alternatives that could effectively reduce subsoil acidity in no-tillage systems.
The trial was conducted between August 2022 and May 2023 in an 8-year-old cherry orchard located in the Los Lagos region, with Regina and Kordia varieties (both on Gisela®6), under drip irrigation. Fertilization and orchard liming were managed according to commercial practices since establishment, including the annual application of agricultural lime to the ridges in doses ranging from 1,000 to 2,000 kg ha-1.
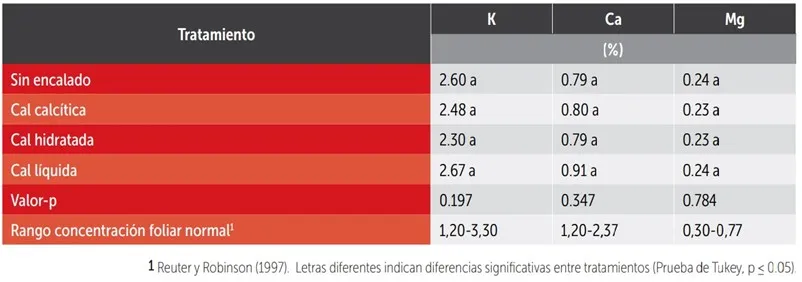 Table 2: Foliar concentration of K, Ca, and Mg according to soil liming treatment in a Kordia/Gisela®6 cherry orchard in the Los Lagos region.
Table 2: Foliar concentration of K, Ca, and Mg according to soil liming treatment in a Kordia/Gisela®6 cherry orchard in the Los Lagos region.
The experiment was conducted on a volcanic soil classified as “Aquic Hapludands,” using a completely randomized block design with three replicates, comprising three treatments with different lime amendments applied at commercial doses to undisturbed soil and a control treatment without lime application (Figure 1; Table 1).
Before applying the treatments and throughout the study period, precipitation was abundant.
Soil and leaf sampling: prior to the start of the experiment, soil samples were collected at a depth of 0–30 cm to analyze their chemical properties. Subsequently, samples were collected at different dates up to 225 days after treatment application was completed. On each date, soil samples were taken from the upper row of each experimental unit at various depths: 0–5 cm, 5–10 cm, and 10–20 cm (Figure 1). The nutritional status of the trees was assessed through foliar analyses of leaf samples taken from the middle third of the shoots and collected in midsummer. All analyses were performed at the PUC Agroanalysis Laboratory.
Results: Agricultural lime applied as mulch gradually increased soil pH during the experimental period within the top 5 cm of depth. Starting 30 days after application, soil pH was significantly higher compared to before liming, with a difference of +0.34 pH units. At the end of the experiment, this difference reached +0.58 pH units (Figure 3a). The effects at greater depths were not significant, making top-dressing lime application ineffective in improving subsoil acidity in the short term (< 1 year), even in areas with high rainfall favoring downward movement of HCO3- and OH- anions from lime dissolution.
The hydrated lime reacted more quickly but had a shorter duration compared to agricultural lime, with a peak in pH observed 15 days after its application within the top 10 cm of depth. Subsequently, pH values decreased over time, although a residual effect of +0.30 pH units remained, but only within the top 5 cm of soil. Therefore, despite the greater solubility of hydrated lime in water, no greater mobility of alkalinity was observed within the soil profile compared to agricultural lime (Figure 2a).
Regarding liquid lime, the applied dose did not result in any changes in soil pH at any depth throughout the experimental period. The applied dose (50 L ha-1) recommended by the manufacturer for orchards in acidic soils was insufficient, as it equates to a dose of 170 kg of pure CaCO3/ha. Additionally, it did not penetrate the soil profile with water, possibly due to its suspension rather than solution nature (Figure 2a).
Unlike soil pH, the response of exchangeable Al to liming treatments was practically null (Figure 2b). This lack of response was expected given the high surface soil pH, as pH levels above 6.0 generate minimal variations in exchangeable Al, with most of it already precipitated.
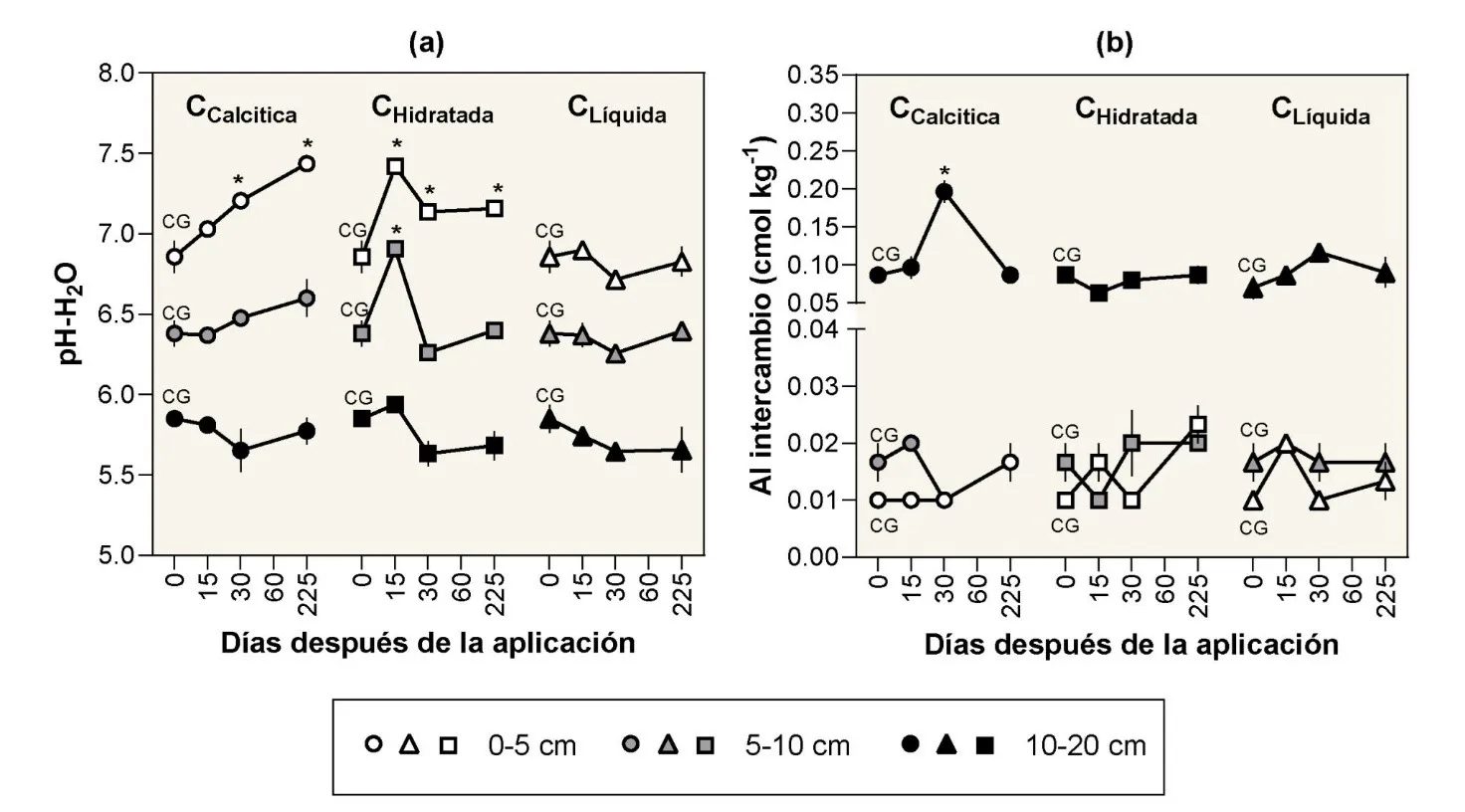 Figure 2: Variation over time (0–225 days) of (a) soil pH-H2O and (b) exchangeable Al at different depths after the application of various liming materials as mulch in a cherry orchard in the Los Lagos region. * Indicates a pH or Al value significantly higher than the control (GC) (Dunnett test, p ≤ 0.05). CCalcitica, agricultural or calcitic lime; Cidratata, hydrated lime; Cliquida, liquid lime.
Figure 2: Variation over time (0–225 days) of (a) soil pH-H2O and (b) exchangeable Al at different depths after the application of various liming materials as mulch in a cherry orchard in the Los Lagos region. * Indicates a pH or Al value significantly higher than the control (GC) (Dunnett test, p ≤ 0.05). CCalcitica, agricultural or calcitic lime; Cidratata, hydrated lime; Cliquida, liquid lime.
Exchangeable Ca in the soil responded similarly to pH, with increases within the top 5 cm of soil after the application of calcitic and hydrated lime, reflecting the low mobility of Ca within the soil profile, enhanced by the high surface pH, which could increase negative charges in variable-charge soil colloids like those in this study.
Considerations on Continuous Lime Applications in Cover Crops
The initial soil analysis, conducted before the experiment installation and based on a sample taken from a depth of 0-30 cm, showed that the studied orchard had soil with a high organic matter content (18%) and adequate levels of P (21 ppm P-Olsen) and K (176 ppm).
However, the soil pH was acidic (5.5) with high levels of exchangeable Al (0.31 cmol kg-1) and Al saturation (8.5%), as well as low availability of Ca (2.46 cmol kg-1) and Mg (0.41 cmol kg-1) (Figure 3).
Conversely, the results of the soil analyses based on high-resolution sampling (0-5, 5-10, 10-20, and 20-30 cm) revealed a marked stratification of acidity variables in the top 30 cm of soil, with a soil pH close to neutrality in the top 5 cm, decreasing to slightly to moderately acidic values between 5 and 20 cm, and reaching a strongly acidic pH at 20-30 cm. On the other hand, Al availability increased with depth (Figure 3a and b), becoming limiting for root growth in the 20-30 cm layer.
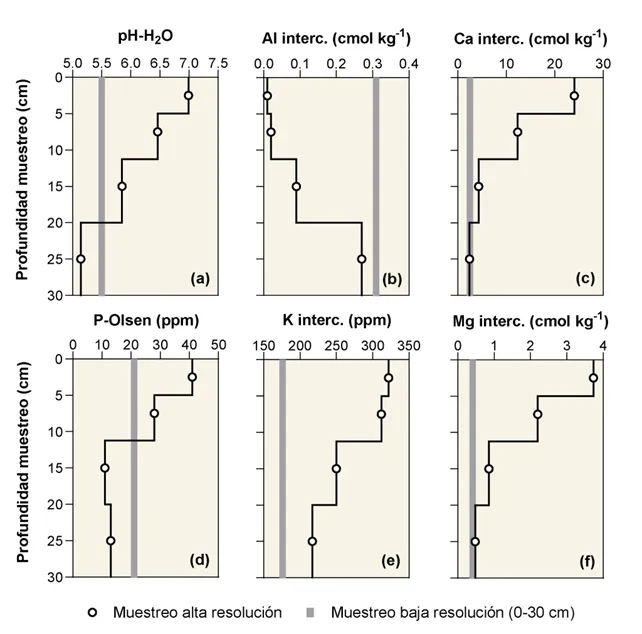 Figure 3: Variation in the first 30 cm of soil depth of (a) pH-H2O, (b) exchangeable Al, (c) exchangeable Ca, (d) available P, (e) exchangeable K, and (f) exchangeable Mg in a cherry orchard in the Los Lagos region with a history of surface lime management. The gray bar indicates the value of soil analysis from a sample taken at 0-30 cm depth.
Figure 3: Variation in the first 30 cm of soil depth of (a) pH-H2O, (b) exchangeable Al, (c) exchangeable Ca, (d) available P, (e) exchangeable K, and (f) exchangeable Mg in a cherry orchard in the Los Lagos region with a history of surface lime management. The gray bar indicates the value of soil analysis from a sample taken at 0-30 cm depth.
This spatial pattern in the soil profile is not natural in young volcanic soils, but is the result of successive broadcast applications of agricultural lime over the past 8 years and represents a widespread situation in orchards on acidic volcanic soils in southern Chile. Another consequence of this lime management type is the accumulation of Ca in the upper soil layers (Figure 3c). In fact, the concentration of Ca in the top 5 cm of soil reached 24 cmol kg-1, double that at 5-10 cm, six times greater than at 10-20 cm, and 12 times greater than at 20-30 cm depth.
According to the concept of balanced basic cation ratios (BCSR), the accumulation of one cation in the soil could cause antagonistic competition in the absorption of other basic cations by plants. To avoid this, an “ideal” soil should have concentrations of Ca, Mg, and K close to 65%, 10%, and 5% of the base sum, respectively. This translates to a Ca/Mg ratio of 6.5:1, Ca/K of 13:1, and Mg/K of 2:1. However, in this study, deviations from optimal values generated by liming treatments did not translate into changes in the trees' nutritional status as determined by foliar analysis (Table 2).
Furthermore, despite the high surface concentration of Ca (Figure 3c), the foliar concentration of Ca was within the deficiency range, and that of K within the normal range, regardless of the liming treatment (Table 2). Therefore, the concept of BCSR might not be directly applicable to fruit trees as to herbaceous crops, especially due to their greater rooting depth that allows them to explore various fertility conditions along the soil profile.
Notably, for sweet cherries on Gisela®6 rootstock, rooting depth can exceed one meter, and depending on soil environmental conditions, root production can concentrate below 25 cm depth (Artacho and Bonomelli, 2016).
Finally, our results also highlight the need to refine soil sampling schemes in orchards.
Sampling schemes with large depth increments ignore the stratification of soil chemical properties resulting from surface fertilization and/or liming, leading to incorrect decisions regarding fertilization or amendments. For example, in the cherry orchard of this study, the results of soil analysis from a sample taken at 0-30 cm depth indicate the need to lime this soil volume (Figure 3a, b, c).
However, when sampling was performed at small depth increments, the need for lime was found below 20 cm depth (Figure 3a and b), ruling out surface lime application as previously done, and equipment should be found or adapted to place lime at depth without damaging the roots.
Similar criteria should be applied for fertilization with immobile (P) and relatively immobile nutrients such as K and Mg. The accumulation of these nutrients in the upper soil layers (41 ppm of P-Olsen, 322 ppm of K, and 3.73 cmol of Mg kg-1) (Figure 3d, e, f) excludes the possibility of mulching with annual maintenance fertilization.
Final Considerations
Successive lime applications, as common management in orchards on volcanic soils in southern Chile, increase soil pH and Ca content only in the top few centimeters of the soil profile, without deepening to reduce acidity and Al3+ toxicity in the layers where the active roots of fruit trees concentrate.
On the other hand, the accumulation of Ca on the soil surface may have caused antagonistic competition in the absorption of other cations. However, the trees had sufficient K uptake rates to meet their demand (foliar analysis). Conversely, foliar Ca concentration was deficient in all cases. This suggests Ca uptake from a deeper soil profile zone where root exploration meets suboptimal soil conditions regarding pH and Ca availability. The results highlight the importance of providing sufficient levels of basic cations throughout the soil profile (rather than seeking "optimal" cation ratios) to ensure adequate uptake, especially considering the deeper rooting depth and root distribution of fruit trees.
Finally, a noteworthy practical aspect emerging from this study: once established, orchards function as no-till cropping systems, where soil sampling becomes more important for accurate nutritional diagnosis, given the surface lime and/or fertilization management.
In this sense, soil sampling with higher resolution should be preferred in the first 20 cm of soil, i.e., with 10 cm depth increments, then increasing to 20-30 cm increments depending on soil stratification.
This approach captures soil property variation at depth, allowing the fruit grower to assess fertilization effectiveness, decide on application frequency, understand actual nutrient supply, and make appropriate fertilization and amendment management decisions.
Of course, all this will be complemented by information from timely foliar analysis. The challenge remains to find the methodology, source, and/or equipment to incorporate lime at depth in no-till cropping systems.
Source: Redagrícola
Pamela Artacho, Claudia Bonomelli
Faculty of Agricultural and Food Sciences, Universidad Austral de Chile, and Faculty of Agronomy and Natural Systems, Pontificia Universidad Católica de Chile.
Cherry Times - All rights reserved















