The behavior of cherries subjected to the application of plant hormones under high temperatures and water restrictions was observed, both in the laboratory and in the field.
Climate change has emerged as a significant threat to global agriculture: rising temperatures and changing rainfall patterns are leading to increased drought stress in many cultivated species (Madeira, 2022).
Among these is the sweet cherry (Prunus avium), grown mainly for its high market value and strong consumer demand. In Chile, known for its optimal climatic conditions for cherry production, the cultivation of specific cultivars such as Sweetheart and Santina is facing considerable challenges related to the impacts of climate change.
Risks and challenges for production
The increasing frequency and intensity of drought events, combined with high temperatures, jeopardize tree physiology, fruit quality, and overall yield, making it urgent to identify effective strategies to adapt cherry production to these stress factors.
Sweet cherry cultivars grown in Chile are particularly sensitive to water deficits and heat stress. Drought can compromise critical physiological processes such as photosynthesis, flowering, and fruit set, leading to reduced yields and lower fruit quality (Pang et al., 2025).
To mitigate the adverse effects of climate change on sweet cherry production, innovative agronomic approaches are essential. A promising strategy is the use of phytohormones, which play a key role in regulating plant responses to abiotic stress.
Role of phytohormones
The use of abscisic acid (ABA), methyl jasmonate (MeJA), and salicylic acid (SA) has shown potential in improving plant resilience and productivity under stress conditions (Shinohara and Leskovar, 2014; Tayyab et al., 2020).
ABA, for example, is essential for regulating stomatal closure in cases of water deficit, thereby improving water use efficiency and maintaining physiological processes under drought stress. Application of ABA in cherries has been associated with better growth and fruit quality, reducing water loss and maintaining turgor pressure (Shinohara and Leskovar, 2014).
MeJA has shown promising effects in increasing resistance to abiotic stresses, with better fruit development and higher yield. Similarly, salicylic acid acts as a key signaling molecule, promoting systemic acquired resistance and improving overall plant health and productivity under stress conditions (Errazúriz-Montanares, 2025).
Management strategies
The integration of these phytohormones into fertigation strategies or foliar applications represents a practical approach to mitigating stress factors and optimizing cherry production in the face of climate change.
The main crop management strategy proposed for cherry is the implementation of controlled deficit irrigation, applied at times when the crop is more tolerant to water shortage, namely in the post-harvest stage.
However, although in the post-harvest stage the plant shows greater tolerance to water shortage, numerous studies have highlighted that summer environmental stress can still have negative impacts on cherry productivity.
Biostimulation and future research
Therefore, the use of chemical strategies or stimulants in the post-harvest period could complement this water management, reducing its negative effects on the crop.
Biostimulation is another management strategy. It is defined as the use of any substance or microorganism whose application increases nutrient use efficiency, generates tolerance to abiotic stress, or improves an agronomic trait.
The main groups of biostimulants are divided into the following categories: beneficial microorganisms (fungi and bacteria), seaweed and plant extracts, hydrolyzed proteins and other nitrogenous compounds, fulvic and humic compounds, chitosan and other biopolymers, inorganic and phytohormonal compounds.
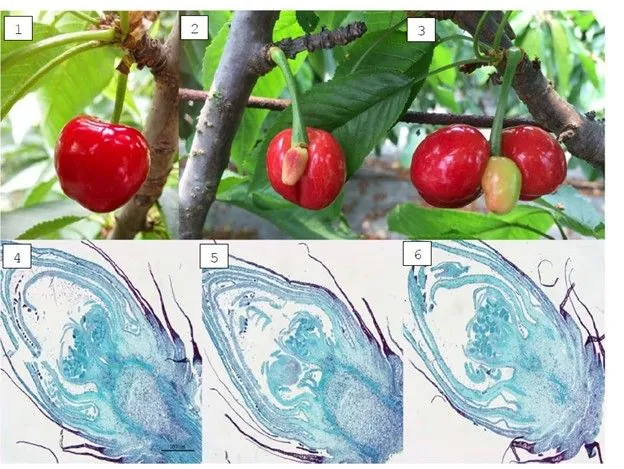 Image 1. Effect of high temperatures on the formation of double fruits and malformations. (Images 1 and 4 correspond to the same fruit; as do 2 and 5, and 3 and 6).
Image 1. Effect of high temperatures on the formation of double fruits and malformations. (Images 1 and 4 correspond to the same fruit; as do 2 and 5, and 3 and 6).
Laboratory and field trials
By analyzing the implications of rising temperatures and drought stress on sweet cherry cultivars in Chile, the aim is to highlight the crucial role of phytohormones as mitigation tools to effectively manage abiotic stress. Future research will need to focus on clarifying the specific mechanisms through which ABA, MeJA, and SA improve stress tolerance and productivity in Sweetheart and Santina cultivars.
By deepening the understanding of these interactions, it will be possible to provide cherry growers with more effective tools to adapt to a changing climate, ensuring sustainable production and the economic profitability of the sector.
During the 2021/2022 season, a study was conducted at the micropropagation laboratory of the Universidad de Talca, in the Maule Region, using 80 sweet cherry plants (Prunus avium) cv. Santina, 30 cm tall and grafted onto ‘Maxma 14’ rootstock.
Laboratory experiments
Leaf soil was used as the substrate and plastic pots as containers, with a volume of 2000 cm³. Before transplanting the plants, the amount of substrate was measured and added to the pots in uniform proportion. After planting, the maximum water holding capacity (in liters per pot) was measured, and during the experiment, the moisture level was monitored, with water restored individually for each pot.
All plants received sufficient water (100% of the pot’s water holding capacity) until October 19, 2021. The eighty plants were randomly distributed in 8 blocks, with 10 plants per block, assigning each plant a treatment with plant growth regulators (PGR).
The treatments were: control without application, abscisic acid (Sigma Aldrich 98% CAS 14375-45-2) at a concentration of 0.44 mM, salicylic acid (Sigma Aldrich 99% CAS 69-72-7) at a concentration of 0.36 mM, and methyl jasmonate (Sigma Aldrich 95% CAS 39924-52-2) at a concentration of 0.37 mM.
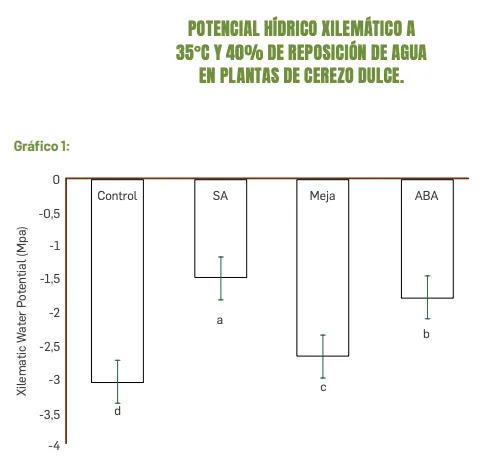 Figure 1. Xylem water potential at 35°C and 40% water replenishment in sweet cherry plants.
Figure 1. Xylem water potential at 35°C and 40% water replenishment in sweet cherry plants.
Conditions and applications
After the applications, the plants were exposed to a combination of temperatures (25°C and 35°C) and irrigation levels (100% and 40% of the pot’s water holding capacity). Applications were made each time before the start of the exposure cycle to the temperature and irrigation combinations.
The plants were maintained under these conditions for 72 hours, followed by 96 hours of recovery before starting the next cycle.
In parallel, during the 2022/2023 season a field study was conducted on two orchards. The first was a sweet cherry cv. Sweetheart/Colt planted in 2017, located at 34°15’19.44”S; 71°0’36.43”W.
Field experiments
The second was a sweet cherry cv. Santina/Maxma 14 planted in 2015, located at 35°22”S; 71°48”W. In both orchards, treatments and measurements were carried out during the summer, between December and February.
The plants grew under commercial conditions; 30 plants per treatment were arranged in randomized blocks. The treatments were: control without applications, abscisic acid (Sigma Aldrich 98% CAS 14375-45-2) at a concentration of 0.44 mM, salicylic acid (Sigma Aldrich 99% CAS 69-72-7) at a concentration of 0.36 mM, and methyl jasmonate (Sigma Aldrich 95% CAS 39924-52-2) at a concentration of 0.37 mM.
Three monthly applications were made during the experiment: in December, January, and February.
Physiological and productive measurements
To characterize the plant’s water status, xylem water potential (Ψx; MPa) was measured using the pressure chamber method (PMS Instrument Co., model 1000, Corvallis, Oregon, USA). A healthy leaf located in the middle third of each evaluated plant was selected, covered with plastic and aluminum foil 1 hour before measurement.
To assess the physiological response of the plants to the different treatments, physiological variables of stomatal conductance (gsw; mol m−2s−1) and net assimilation rate (An; µmol m−2s−1) were measured using a portable gas exchange analyzer (Li-6800 LI-COR).
In the case of the field experiment, yield per treatment in the current season was also measured.
Results
Under optimal laboratory conditions (25°C and 100% irrigation), no significant differences were found between treatments. Stomatal conductance (GSW) values ranged between 0.08 and 0.16 mol m−2 s−1.
When plants were subjected to water restriction, conductance decreased significantly in the control, ABA, and MeJA treatments to values of 0.06 mol m−2 s−1, while the SA treatment maintained the same conductance values recorded under previous conditions.
When plants were exposed to heat stress (35°C) but with full water supply (100% replenishment), the control and MeJA treatments reduced conductance to values of 0.07 mol m⁻² s⁻¹, while SA showed the highest values (0.12 mol m⁻² s⁻¹) and the ABA treatments recorded the lowest values (0.01 mol m⁻² s⁻¹).
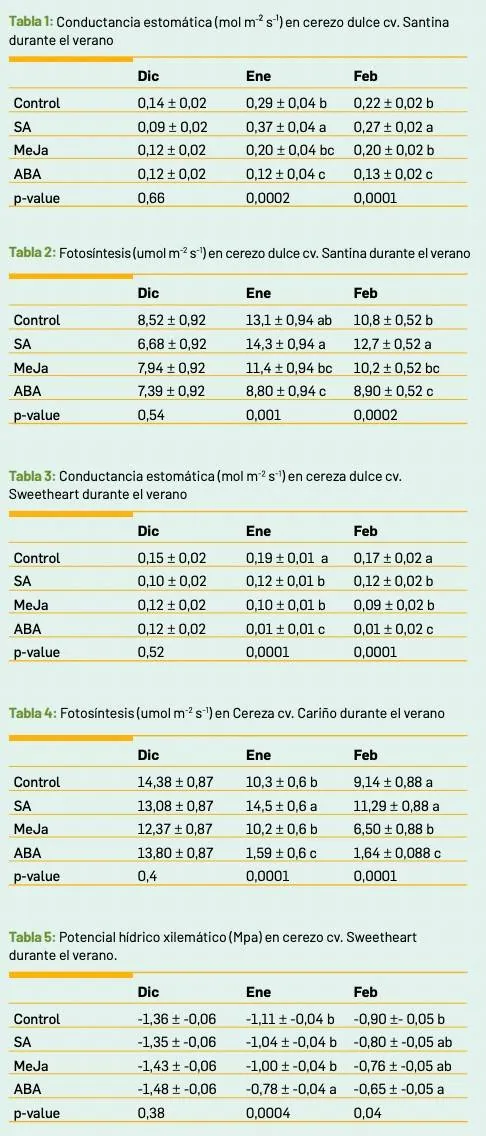 Table 1: Stomatal conductance (mol m² s⁻¹) in sweet cherry cv. Santina during summer.
Table 1: Stomatal conductance (mol m² s⁻¹) in sweet cherry cv. Santina during summer.
Table 2: Photosynthesis (μmol m² s⁻¹) in sweet cherry cv. Santina during summer.
Table 3: Stomatal conductance (mol m² s⁻¹) in sweet cherry cv. Sweetheart during summer.
Table 4: Photosynthesis (μmol m² s⁻¹) in cherry cv. Cariño during summer.
Table 5: Xylem water potential (MPa) in sweet cherry cv. Sweetheart during summer.
Conclusions
When plants were subjected to high temperatures (35°C) and a reduction in water replenishment (40% of requirement), all treatments showed the same GSW value, with values below 0.02 mol m⁻² s⁻¹.
In conclusion, the application of phytohormones to prevent or mitigate heat stress and drought in sweet cherries could represent a useful tool to help growers maintain productivity under unfavorable environmental conditions related to climate change.
SA, MeJA, and ABA impact the physiology and functioning of sweet cherry plants both under controlled conditions and in commercial contexts.
Source text and internal images: mundoagro.io
Cover image source: Francisco Maldonado, University of Talca (Chile)
Francisco Maldonado
University of Talca (Chile)
Cherry Times - All rights reserved













